| Product No. | SA029 |
|---|---|
| CAS Reg. No. | 122-16-7 |
| Alternate CAS Reg. No. | - |
| Offer | 250 mg |
122-16-7
- Documentation
- Details
Chemical name
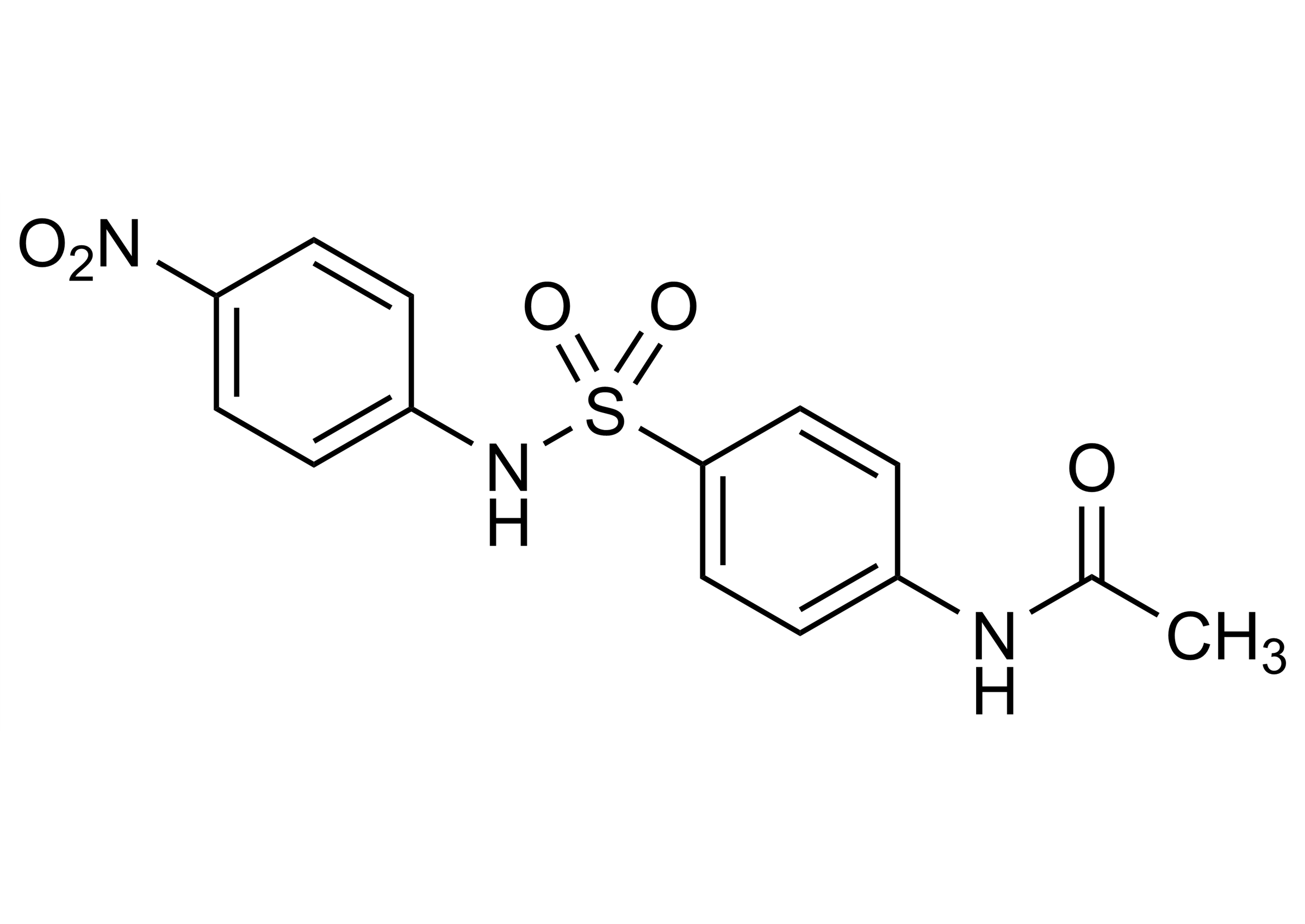
N-[4-[[(4-Nitrophenyl) amino] sulfonyl]phenyl] acetamide
Description
Sulfanitran (CAS 122-16-7) reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH supports robust LC-MS/MS and GC-MS quantification. This sulfonamide residue standard enables reliable calibration, clear traceability, and decisive confirmatory analysis. Laboratories use it to build defensible methods with consistent performance across matrices.
Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH, this high-purity material helps you streamline method development. You can establish linear calibration curves, verify instrument response, and assess recovery. Moreover, you can document traceability for audits and routine quality control. Consequently, your data remains reproducible and comparable over time.
Typical applications include:
- Regulated laboratory residue monitoring and compliance testing
- Pharmaceutical research and metabolism studies
- Residue control in food and feed surveillance
- Multi-residue method development and optimization
- Routine QC checks and proficiency testing
Use this reference standard to tighten measurement uncertainty and support method validation steps. It fits LC-MS/MS and GC-MS workflows, including matrix-matched calibration and isotope-free quantitation. In addition, it aids selectivity studies, recovery experiments, and instrument suitability checks. Therefore, you can confirm identity with characteristic transitions or ions and quantify confidently.
Each batch features consistent quality for traceable calibration and precise quantification. You receive lot-specific documentation to support accreditation needs and reporting. Furthermore, practical usage is straightforward:
- Prepare gravimetric stock solutions in suitable solvents
- Build calibration levels that match expected ranges
- Evaluate linearity, accuracy, precision, and LOQ
- Confirm analyte identity with orthogonal criteria
This Sulfanitran reference standard strengthens analytical reliability from screening to confirmatory analysis. Additionally, it supports rugged, compliant workflows across instruments and matrices, backed by WITEGA expertise.
Safety Data Sheet
You can download your Safety Data Sheet for SA029
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | N-[4-[[(4-Nitrophenyl) amino] sulfonyl]phenyl] acetamide |
|---|---|
| Molecular Formula | C14H13N3O5S |
| Molecular Weight | 335.34 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | SA029 |
| CAS Reg. No. | 122-16-7 |
| Alternate CAS Reg. No. | – |
| Offer | 250 mg |